Background: With the incorporation of positron emission tomography (PET) imaging as part of the standard staging evaluation of follicular lymphoma (FL), it is generally recommended to obtain the diagnostic biopsy from a lesion with the highest standardized uptake value (SUV) to rule out de novo histologic transformation (HT). In some cases such an approach might be impractical, while in other cases a biopsy from a diseased area with a high SUV may still demonstrate an indolent histology. To date, there is no data to guide treatment choices in patients (pts) with FL with high SUVmax but with no documented evidence of HT. Specifically, it remains unclear whether anthracycline-containing regimens, such as R-CHOP, provide a better outcome than R-Bendamustine (BR). Furthermore, it is unknown whether rituximab (R) maintenance is beneficial in this setting. Therefore, we aimed to compare the efficacy of R-CHOP vs BR in newly diagnosed FL pts with high SUVmax at baseline PET and to clarify the role of maintenance.
Patients and Methods: We retrospectively identified 261 consecutive pts with newly diagnosed biopsy-proven FL1-3A and a SUVmax≥13 on baseline PET, who were treated with frontline R-CHOP (n=183) or BR (n=78) at 7 US cancer centers based on the physician's choice. Progression-free survival (PFS) was calculated from starting treatment until progression, relapse or death. Cut-offs of SUVmax ≥13≤18 and SUVmax>18 were used for sub-analysis (Haematologica;2020;105:1907-1913).
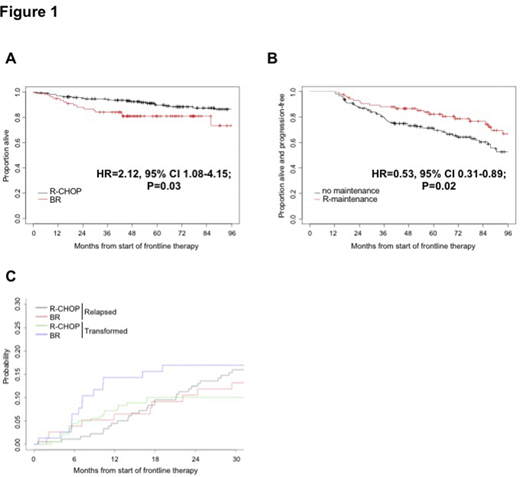
Results: Median age was 59 years. The baseline characteristics of the two groups differed significantly for a younger age (58 vs 62 years, p=0.009), a higher rate of B-symptoms (26% vs 10%, p=0.005) and baseline SUVmax>18 (49% vs 36%, p=0.04) in the R-CHOP group. A diagnostic biopsy was obtained from the site at the highest SUV in 129 (71%) and 43 (55%) pts, respectively (p=0.01). These suggest a potential selection of R-CHOP in pts with adverse features. At the end of therapy (EoT), R-CHOP treated pts achieved higher PET-CT complete response rates (CR 82% vs 69%, p=0.02). This superiority held in pts with diagnostic biopsy at the highest SUV site (CR 83% vs 67%, p=0.03), but not in the others (CR 80% vs 71%, p=0.37). Progression occurred at EoT in 11 and 14 pts receiving R-CHOP and BR (6% vs 18%, p=0.02), with a higher rate of HT in the BR group (13% vs 4%, p=0.006). After a median follow-up of 76 months (range, 4-208 months), there was no significant difference in PFS between R-CHOP and BR treated pts (hazard ratio [HR] 1.22, p=0.34). PFS was strongly associated with FLIPI 2 (HR 2.32, p=0.01) and 3-5 (HR 2.69, p=0.003) in the univariate as well as in the multivariate analysis (FLIPI 2 HR 2.19, p=0.02; FLIPI 3-5 HR 2.51, p=0.01). There was a trend toward overall survival (OS) benefit in the R-CHOP group (Fig.1A). In the univariate analysis for OS, BR regimen (HR 2.12, p=0.03), age >60 (HR 2.54, p=0.006), FLIPI 3-5 (HR 5.13, p=0.03) and biopsy at the highest SUV site (HR 0.44, p=0.01) were prognostic, however none of those remained significant in the multivariate analysis (BR regimen HR 1.84, p=0.09; age >60 HR 1.92, p=0.08; FLIPI 3-5 HR 3.11, p=0.14; biopsy at the highest SUV site HR 0.60, p=0.13; SUVmax>18 HR 1.87, p=0.055).
Among pts with EoT response after R-CHOP or BR (n=170 and n=64, respectively), one third in each group (36% vs 41%, respectively) received R-maintenance for a median of 8 administrations (range, 2-12). A significant PFS advantage (landmark analysis) was observed with R-maintenance (Fig.1B; HR 0.53, p=0.02); however, this did not translate in survival benefit (HR 0.67, p=0.49). In a multivariable model, R-maintenance (HR 0.53, p=0.02), FLIPI 2 (HR 2.47, p=0.03) and 3-5 (HR 2.79, p=0.01) remained independent prognosticators of PFS, while no parameters significantly affected OS. The 2-year HT rate (10% vs 17%) and cumulative incidence did not statistically differ between R-CHOP and BR groups (Fig.1C, p=0.159).
Early progression (POD24) occurred in 43 and 22 pts who received R-CHOP and BR, respectively (24% vs 28%) and resulted in a significantly higher risk of death (HR 4.12, p=0.006).
Conclusion: In newly diagnosed FL pts with SUVmax≥13 at baseline PET, R-CHOP was more effective in achieving CR and reducing the risk of early HT compared with BR, and it was associated with a trend toward survival advantage. Rituximab maintenance significantly prolonged PFS. A prospective evaluation with a larger population will be needed to confirm our findings.
Joffe:Epizyme: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Membership on an entity's Board of Directors or advisory committees. Ruella:Abclon, BMS, NanoString: Consultancy; Abclon: Consultancy, Research Funding; UPenn/Novartis: Patents & Royalties. Svoboda:Adaptive: Consultancy; Astra-Zeneca: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Imbrium: Consultancy; Incyte: Research Funding; Pharmacyclics: Consultancy, Research Funding; Merck: Research Funding; Seattle Genetics: Consultancy, Research Funding; TG: Research Funding; Genmab: Consultancy; Atara: Consultancy. Witzig:Immune Design: Research Funding; Spectrum: Consultancy; Karyopharm Therapeutics: Research Funding; Acerta: Research Funding; Celgene: Consultancy, Research Funding; AbbVie: Consultancy; MorphSys: Consultancy; Incyte: Consultancy. Smith:TG Therapeutics: Consultancy, Research Funding; Janssen: Consultancy; BMS: Consultancy; Celgene: Consultancy, Research Funding; Pharmacyclics: Research Funding; Karyopharm: Consultancy, Research Funding; FortySeven: Research Funding; Acerta: Research Funding; Genentech/Roche: Consultancy, Other: Support of parent study and funding of editorial support, Research Funding. Armand:Affimed: Consultancy, Research Funding; Otsuka: Research Funding; Bristol-Myers Squibb: Consultancy, Honoraria, Research Funding; IGM: Research Funding; Infinity: Consultancy; Genentech: Research Funding; Sigma Tau: Research Funding; Celgene: Consultancy; Adaptive: Consultancy, Research Funding; Tensha: Research Funding; Roche: Research Funding; Merck & Co., Inc.: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy; ADC Therapeutics: Consultancy. Nastoupil:Janssen: Honoraria, Research Funding; LAM Therapeutics: Research Funding; Gilead/KITE: Honoraria; Gamida Cell: Honoraria; TG Therapeutics: Honoraria, Research Funding; Merck: Research Funding; Novartis: Honoraria, Research Funding; Genentech, Inc.: Honoraria, Research Funding; Karus Therapeutics: Research Funding; Bayer: Honoraria; Celgene: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding. Ansell:Takeda: Research Funding; Regeneron: Research Funding; Bristol Myers Squibb: Research Funding; Seattle Genetics: Research Funding; Trillium: Research Funding; Affimed: Research Funding; AI Therapeutics: Research Funding; ADC Therapeutics: Research Funding. Zelenetz:Novartis: Consultancy; Adaptive Biotechnology: Consultancy; Genentech/Roche: Consultancy; Gilead: Consultancy; Gilead: Research Funding; Celgene: Consultancy; Roche: Research Funding; Amgen: Consultancy; BeiGene: Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy; Sandoz: Research Funding; Celgene: Research Funding; MorphoSys: Research Funding; MEI Pharma: Research Funding. Younes:BioPath, Xynomic, Epizyme, and F. Hoffmann-La Roche: Consultancy; Janssen, Curis, Merck, Bristol-Myers Squibb, Syndax Pharmaceuticals, F. Hoffmann-La Roche, Curis (Inst), Johnson & Johnson (Inst), Novartis (Inst): Research Funding; Janssen, AbbVie, Merck, Curis, Epizyme, F. Hoffmann-La Roche, Takeda, Bristol-Myers Squibb, Bayer HealthCare Pharmaceuticals, Celgene, Incyte, Janssen Pharmaceuticals, Merck, Sanofi, Seattle Genetics, Takeda Millennium: Honoraria; AstraZeneca: Current Employment; MSKCC: Ended employment in the past 24 months.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal